Page 9 - Cannabis for the Management of Pain: Assessment of Safety Study
P. 9
Ware et al The Journal of Pain 1241
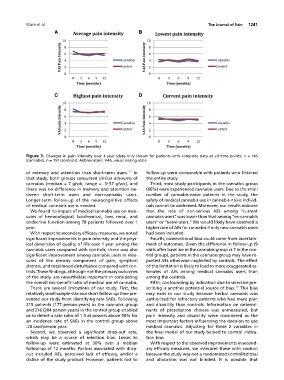
Figure 3. Changes in pain intensity over 1 year (data only shown for patients with complete data at all time points; n = 145
(cannabis), n = 157 (controls). Abbreviation: VAS, visual analog scale.
of memory and attention than short-term users. 11 In follow-up were comparable with patients who finished
that study, both groups consumed similar amounts of the entire study.
cannabis (median = 7 g/wk, range = .3–57 g/wk), and Third, most study participants in the cannabis group
there was no difference in memory and attention be- (66%) were experienced cannabis users. Due to the small
tween short-term users and non-cannabis users. number of cannabis-naive patients in the study, the
Longer-term follow-up of the neurocognitive effects safety of medical cannabis use in cannabis-naive individ-
of medical cannabis use is needed. uals cannot be addressed. Moreover, our results indicate
We found no impact of medical cannabis use on mea- that the rate of non-serious AEs among ‘‘current
sures of hematological, biochemical, liver, renal, and cannabis users’’ was lower than that among ‘‘ex-cannabis
endocrine function among 78 patients followed over 1 users’’ or ‘‘naive users.’’ We would likely have observed a
year. higher rate of AEs for cannabis if only new cannabis users
With respect to secondary efficacy measures, we noted had been included.
significant improvements in pain intensity and the phys- Fourth, observational bias could come from ascertain-
ical dimension of quality of life over 1 year among the ment of outcomes. Given the difference in follow-up (9
cannabis users compared with controls; there was also visits after baseline in the cannabis group vs 7 in the con-
significant improvement among cannabis users in mea- trol group), patients in the cannabis group may have re-
sures of the sensory component of pain, symptom ported AEs otherwise neglected by controls. The effect
distress, and total mood disturbance compared with con- of this limitation is likely to lead to more exaggerated es-
trols. These findings, although not the primary outcomes timates of AEs among medical cannabis users than
of the study, are nevertheless important in considering among the controls.
the overall risk-benefit ratio of medical use of cannabis. Fifth, confounding by indication due to selective pre-
There are several limitations of our study. First, the scribing is another potential source of bias. 15 This bias
relatively small sample size and short follow-up time pre- may exist in our study because herbal cannabis was
vented our study from identifying rare SAEs. Following authorized for refractory patients who had more pain
215 patients (177 person-years) in the cannabis group and disability than controls. Information on determi-
and 216 (204 person-years) in the control group enabled nants of prescription choices was unmeasured, but
us to detect a rate ratio of 1.5 at powers above 50% for pain intensity and disability were considered as the
an incidence rate of SAEs in the control group above most important factors influencing the decision to use
.20 case/person-year. medical cannabis. Adjusting for these 2 variables in
Second, we observed a significant drop-out rate, the final model of our study helped to control indica-
which may be a source of selection bias. Losses to tion bias.
follow-up were estimated at 30% over a median With respect to the observed improvements in second-
follow-up of 12 months. Factors associated with drop- ary efficacy measures, we interpret these with caution
out included AEs, perceived lack of efficacy, and/or a because the study was not a randomized controlled trial
dislike of the study product. However, patients lost to and allocation was not blinded. It is possible that